We’ve supported the discovery, development and launch of hundreds of antimicrobial agents.
IHMA provides a full suite of technical capabilities and domain expertise in all phases of antimicrobial drug development: drug discovery, clinical development, regulatory approval and commercialization.
Why IHMA?
IHMA is a premier provider of antimicrobial drug development studies. With laboratories in both the US and Europe, we partner with clients around the world in the biotechnology, pharmaceutical, and diagnostic industries. We are a leading, independent laboratory specializing in surveillance studies and clinical trials, key milestones along the continuum from drug development to commercialization. We utilize state of the art technology, leading to first-class testing and data as well as sound economic frameworks. Our services can be customized to best align with our clients’ unique testing needs. This ensures successful drug development, commercialization, and post-marketing pathways.
Key Statistics
1,500 + Health Care Institutions Across More Than 80 Countries
Our relationships with hundreds of clinical sites across the world greatly facilitates our ability to conduct antimicrobial resistance surveillance so critical to a drug’s clinical development and commercialization and also supports our central laboratory microbiology services needed to conduct clinical trials.
100s of Thousands of Clinically Relevant Bacterial Isolates
Our Bacterial Repository is an expanding collection of clinically relevant pheno- and genotypically characterized bacterial pathogens. The organisms are catalogued according to a variety of key parameters and can be used to rapidly assemble custom collections to profile the anti-bacterial activities of any products in your discovery pipeline.
Over 1,000 Scientific Presentations and Publications
Our team of experienced clinical microbiologists and scientists have authored hundreds of abstracts, posters, slide presentations, peer-reviewed journal publications, and web accessible data and analysis that are so critical to educating the medical world about any new product.
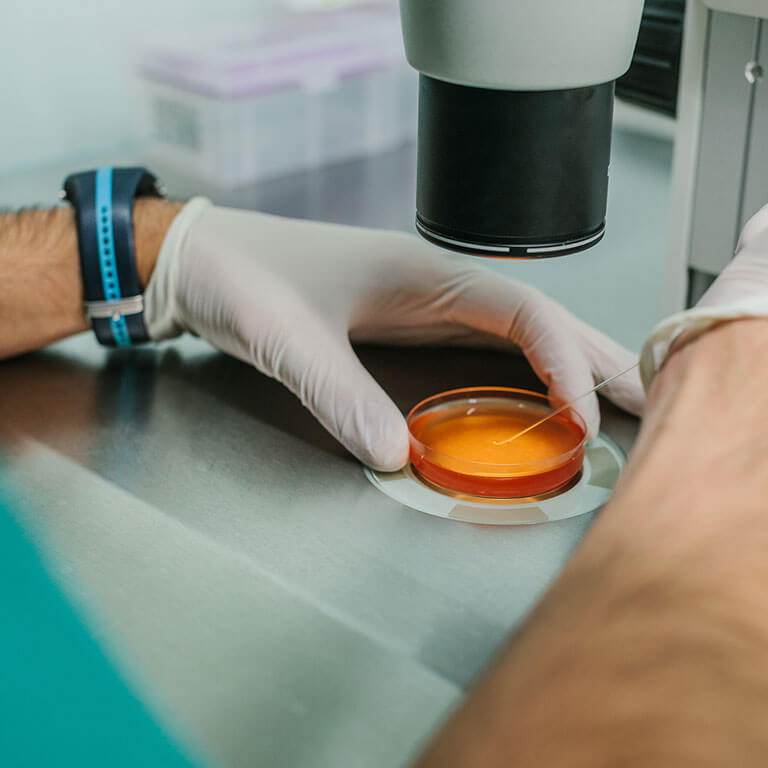
Our fully integrated approach matters.
We provide a fully integrated programmatic approach to support all phases of drug development. This full spectrum of capabilities greatly facilitates client management efforts, provides access to globally available and fully harmonized facilities, and ensures one scientific team is providing access to in depth scientific expertise and ongoing project support.

We’re proud of our story
2022 marks our 30th anniversary. After spending two decades in the pharmaceutical industry in a variety of roles, Jack Johnson founded IHMA in 1992 to help pharmaceutical companies meet the challenges of the dynamic drug development landscape. Today, IHMA is one of the world’s leading providers of antimicrobial drug development support including emerging technology and services. Our team offers complete collaboration and flexibility with all of our clients around the world ensuring ongoing development success.










